DPC
Excipients for nasal spray preparations
In recent years, nasal drug delivery systems have become a research hotspot in the field of pharmaceuticals both at home and abroad due to their advantages such as rapid onset, high bioavailability, convenient administration, central targeting, and absence of liver first-pass effect. However, the absorption process of nasal administration has two main limiting factors: one is the low membrane permeability of the drug, and the other is the clearance effect of nasal mucosal cilia. Currently, the most commonly used method to increase the absorption of drugs in the nasal mucosa is to add absorption enhancers. Absorption enhancers are a type of functional excipient in the formulation prescription, which can improve the transmembrane absorption of active drugs, thereby increasing bioavailability and enabling the drug to exert its maximum therapeutic effect.
Absorption enhancer
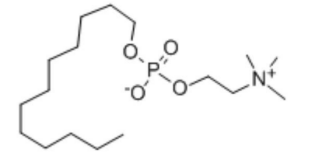
DPC
Dodecylphosphocholine
Synonym: FOS-Vitamin B-12
Abbreviation: DPC
CAS No.:29557-51-5
Molecular formula:C17H38NO4P
Molecular weight:351.46
Mechanism of action: DPC can alter the permeability of the mucosa and enhance the absorption of drug molecules in the body.
molecular structure of phosphatidylcholine
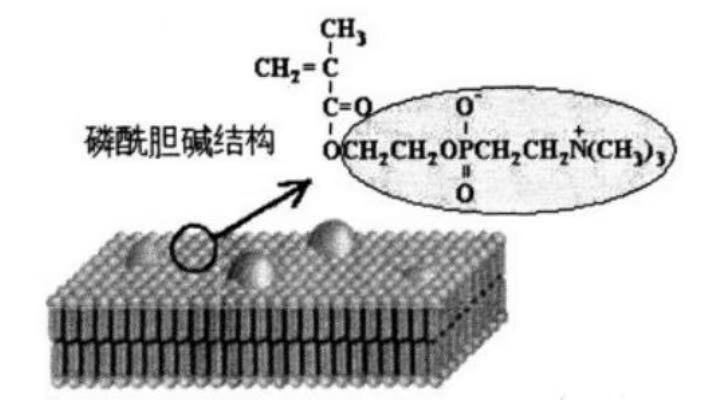
In the molecular structure of phosphatidylcholine, one part consists of hydrophobic non-polar long carbon chains, while the other part is the hydrophilic polar dipole ion, namely the phosphatidylcholine group. When placed in water, the polar group points towards the water surface, and the hydrophobic part aggregates due to the repulsive effect on water, separating from the water and forming a so-called phospholipid-like bilayer, which is extremely similar to the structure of the cell membrane and has excellent biocompatibility.
product application
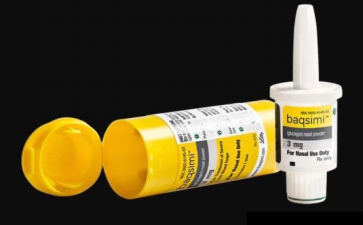
Glucagon Nasal Powder (Baqsimi) is developed by Eli Lilly and Company. It is used for the emergency treatment of severe hypoglycemia in diabetic patients aged 4 years and above. It was approved by the FDA in July 2019 and approved for marketing in the European Union in December 2019. Baqsimi is a powder administered via the nasal route, contained in a single-use spray device. It is the first glucagon therapy for the treatment of severe hypoglycemia that does not require injection.The final product of nasal glucagon is a ready-to-use (no reconstitution required) dry powder formulation. It can be absorbed through the nasal mucosa and serves as an alternative to injectable lyophilized-reconstituted formulations.
The final pharmaceutical product is a nasal powder, containing 3mg of glucagon as the active ingredient, and other excipients including β-cyclodextrin, dodecylphosphatidylcholine (DPC), and acetic acid.This formulation is packaged in single-dose containers, with packaging materials including polyethylene and polypropylene.
Among them, β-cyclodextrin acts as a filler, which can help the powder adhere to the surface of the nasal mucosa, thereby promoting the absorption of glucagon.The reason for choosing β-cyclodextrin is that compared with α-cyclodextrin and γ-cyclodextrin, β-cyclodextrin has the lowest solubility, so it has the longest adhesion time.As a phospholipid-based surfactant, DPC can form micelles in solution, thereby encapsulating glucagon.The reason for choosing DPC is that other phospholipid materials, such as DLPG and DDPC, may also form bilayer liposomes during the micelle formation process, which leads to a decrease in the encapsulation efficiency of glucagon.Finally, acetic acid, as a pH adjuster, can be used to increase the solubility of glucagon in water (pH ≤ 4) and increase the drug concentration, thereby shortening the time required for the subsequent drying process.
Advantages
Mature technology
The analytical detection methods are mature.
Does not contain any patent information and there is no infringement issue.
The kilogram-level prototype has been completed.
DPC, as a penetration enhancer, has demonstrated significant penetration-promoting effects in the high glucose nasal spray formulation of a major product. It is expected to be applied in other large-molecule drug delivery systems as a penetration enhancer for non-invasive administration methods such as oral and nasal spray administration.
CDE registration is in progress. Free samples are available!

