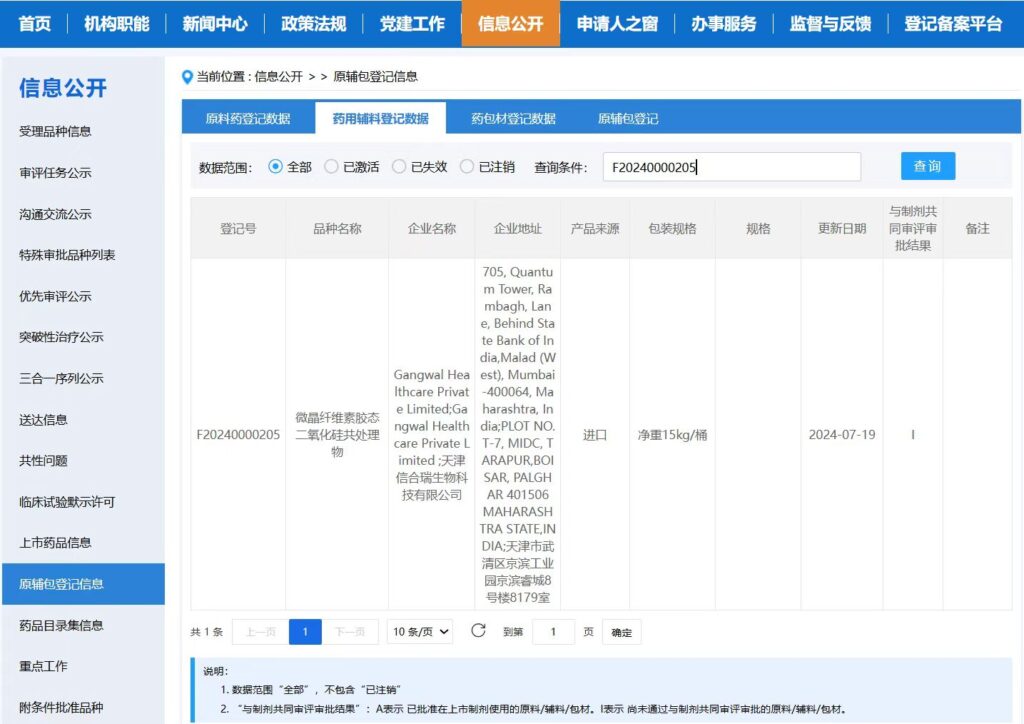
Tianjin XHR Biological Technology Co., Ltd. agent sales for solid preparation of high-quality filler, lubricant – SMCC, its registration number F20240000205 has been published in CDE, welcome to apply for sample !
ProBlend®

ProBlend® SMCC refers to microcrystalline cellulose deposited or adhered to micropowdered silica gel. In 2016, China Food and Drug Administration issued the Requirements for Application Materials for Related Examination and Evaluation of Pharmaceutical Excipients, where it is defined as mixed excipients (jointly treated excipients), which has been included in the 2020 edition of Chinese Pharmacopoeia, and its functions are fillers and lubricants.
SMCC has a unique processing performance, but also has the particularity of both co-aggregation: good hygroscopic resistance, high expansion and compressibility. This co-treatment not only improves the surface performance and fluidity of the excipients, but also overcomes the challenge of poor API compressibility, thus optimizing the performance of the chip core and helping you solve various formulation problems.
Siliconization reduces the adhesion (cohesion) between the powder mixture, thus improving the intrinsic mobility of the powder. It also enhances the surface characteristics of MCC and significantly improves the compressibility and binding force. These properties make it have excellent pharmaceutical excipients processing, solve some wet preparations and process problems with excessive content, excessive type and quantity of excipients, complex preparation process, high production cost, poor patient compliance, especially it can be used in the preparation of such as Chinese medicine dispersed tablets, oral disintegration tablets and other new dosage forms.
ProBlend® SMCC product specifications
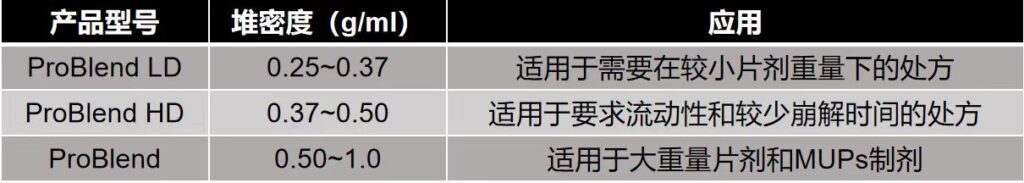
ProBlend® SMCC Product Advantages
★Excellent compressibility and low fragility
★Good liquidity
★Better lubrication efficiency
★Hybrid performance enhancement
★Better mixing uniformity and content uniformity
★Faster manufacturing through direct compression to speed up production
★Clean operation, no dust production
Application
Inactive ingredients:
Colloidal silica, CMC-NA , hydroxypropyl methylcellulose acetate succinate (HPMC AS), magnesium stearate, microcrystalline cellulose, SMCC

Inactive ingredients: cross-linked with carboxyylcellulose sodium, lactose monohydrate, magnesium stearate, polysorbate 20, povidone K30,SMCC

Inactive ingredients: talc powder, CMC-NA, SMCC, magnesium stearate

