Sulfobutylether-β-Cyclodextrin CDE registration number was activated as “ A” state.
Tianjin XHR Biotechnology Co.,LTD agents sales of Sulfobutylether-β-Cyclodextrin, its CDE registration number has been activated, welcome to apply for sample trial!
product presentation
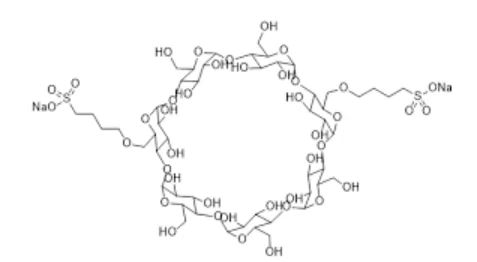
Sulfobutylether-β-Cyclodextrin, It is an anionic, highly water-soluble β-cyclodextrin derivative successfully developed by the American Cydex Company in the 1990s. It is not a single compound component, but is composed of a variety of polymers with different substitution degrees and position/region isomers. It can be well integrated with drug molecules to form non-covalent complexes, so as to improve the stability, water solubility and safety of drugs. Reducing nephrotoxicity, alleviating drug hemolysis, controlling drug release rate, masking bad odor, etc. It has better water solubility, less hemolysis and low nephrotoxicity. It is a new pharmaceutical with a very broad application prospect.
SBECD,It is a multi-substituted derivative of natural β-cyclodextrin (β-CD). It is not a single compound component, but consists of a variety of polymers with different substitution degrees and position/region isomers, and its components are mostly 1, 4, and 7 substitutes.SBECD is mainly used in pharmaceutics to increase drug solubility, improve drug stability, promote drug absorption, reduce drug stimulation to the body, and can also be applied to sustained-release and controlled-release preparations and targeted preparations. Compared with β-CD, SBECD has stronger inclusion ability and solubilization, and higher safety, which overcomes the limitations of β-CD, such as low water solubility and kidney injury.When SBECD is used as an excipient for some drugs, it can reduce the toxic effects of drugs and improve the safety of drugs in vivo. In conclusion, SBECD has incomparable advantages over other cyclodextrin derivatives in terms of physicochemical properties and in vivo safety, and is a promising excipient.
product feature
Sulfobutylether-β-Cyclodextrin is a white powder, odorless, non-toxic, slightly sweet, highly soluble in water; the molecule has a cylindrical shape, with a hydrophobic interior and a hydrophilic sulfobutyl ether substituent on the outside. The hydrophobic interior and the alkyl ether part of the side chain can accommodate hydrophobic drug molecules well, allowing for good entrapment of the drug molecules and the formation of non-covalent complexes with the drug molecules, thus improving the stability, water solubility, safety, reducing nephrotoxicity, mitigating hemolysis, controlling the release rate of the drug, and masking unpleasant odors.
product application
Sulfobutylether-β-Cyclodextrin is a dispersion agent, can also be used as a solubilizer, wetting agent, chelating agent (complexing agent), and multivalent masking agent. As a pharmaceutical excipient, it can be used in injectable drugs, oral drugs, nasal drugs, and eye drugs. It has a special affinity and entrapment for nitrogen-containing drugs and is widely used.
parenteral formulations
Sulfobutylether-β-Cyclodextrin ,It is mainly used in injectable drugs to improve the solubility of poorly soluble drugs, so that the drug can be quickly delivered and reach the required drug volume during injection, reduce the irritation of injection drug on the administration site, improve the stability of the drug in solution, alleviate the blood melting effect of the drug, and improve the safety of drug use.
oral formulation
Increase the stability of drugs or prolong the release time of drugs in the gastrointestinal tract, reduce the irritation of drugs to local tissues, and mask the unpleasant odor of some drugs by modifying the release sites of drugs.
ophthalmic formulations
The application of sodium sulfobutyl ether beta-cyclodextrin in nasal drug delivery mainly increases the permeability of the nasal mucosa, enhances the solubility and stability of drugs, and improves the metabolic rate of drugs at the target site of drug administration.
Application of dosage forms
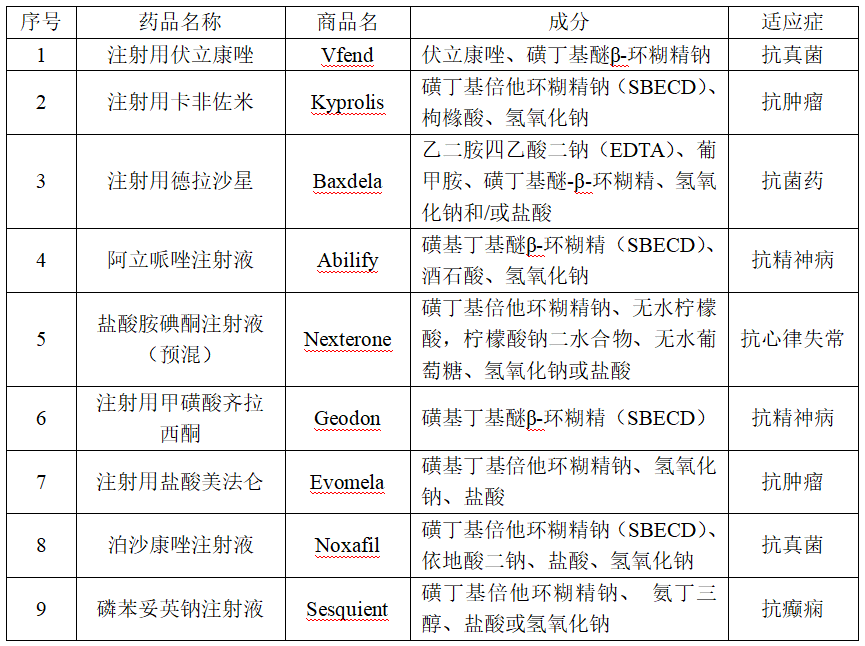
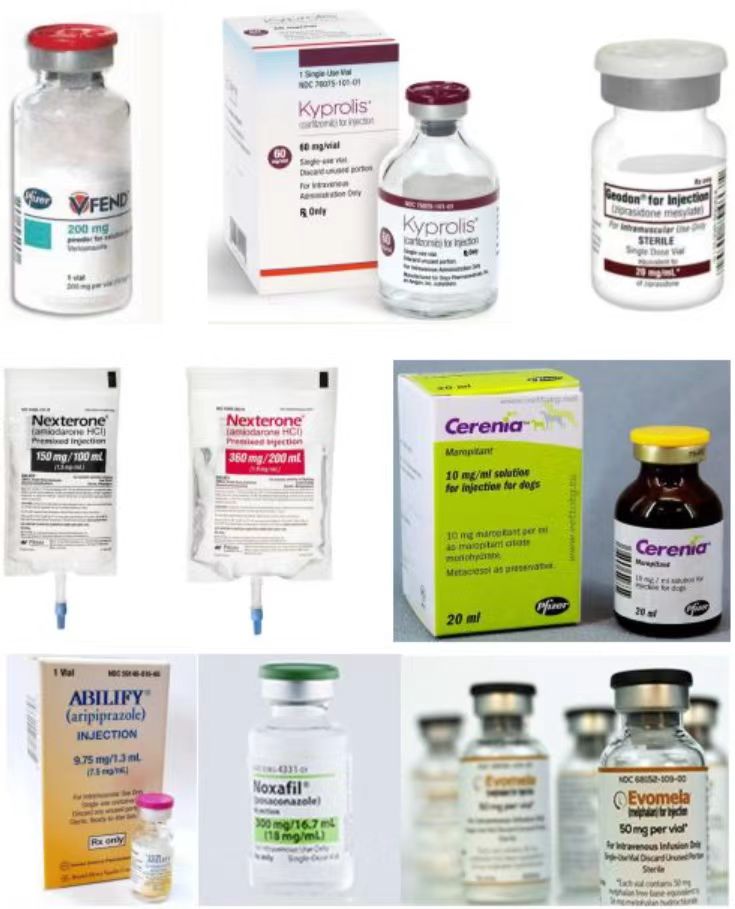
Product Advantage
The product quality conforms to USP and EP standards
The lower limit of impurity B and the lower limit of endotoxin are conducive to the wider use of different preparations
According to customer needs to provide different pH values, different degrees of substitution products
Have a special production line, stable supply; Annual output of more than 100 tons
In China, the United States and Europe and other markets have been registered
① The CHINA registration number and the preparation were jointly reviewed and approved (A)
② The US DMF number is 037814 ( State : A)
③ CEP 2022-504-Rev 00 :European Certificate Number: CEP 2022-504-Rev 00

