Notice on publicly soliciting opinions on the “Guidelines for the Acceptance and Review of Consistency Evaluation of the Quality and Efficacy of Generic Drugs (Draft for Solicitation of Comments)”.
Release date: 20230925
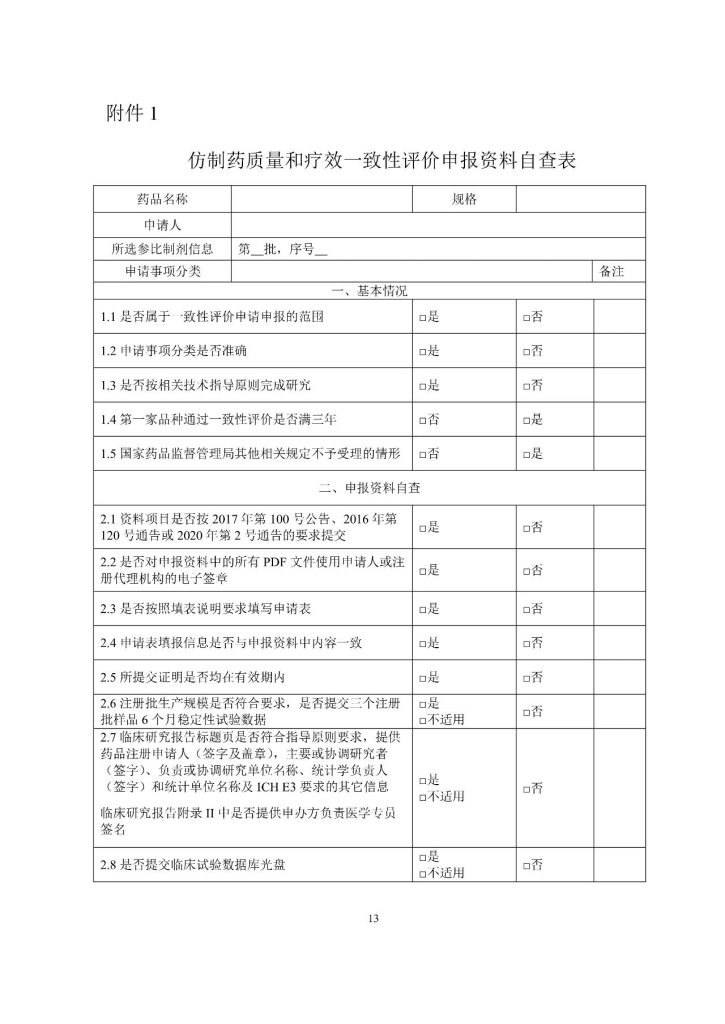
In order to implement the requirements of the reform of the drug review and approval system, and implement the relevant provisions of the Drug Administration Law, the Measures for the Administration of Drug Registration, and the Measures for the Administration of Post-marketing Changes of Drugs (for Trial Implementation), the Drug Evaluation Center issued the “Guidelines for the Acceptance and Review of the Consistency Evaluation of the Quality and Efficacy of Generic Drugs (Varieties Requiring Consistency Evaluation)” and the “Guidelines for the Acceptance and Review of the Consistency Evaluation of the Quality and Efficacy of Generic Drugs (Varieties Produced in China and Marketed in Europe, America and Japan)” (No. 148 of 2017), combined with the relevant requirements of electronic drug registration declaration and consistency evaluation of injections, the Guidelines for the Acceptance and Review of Consistency Evaluation of Quality and Efficacy of Generic Drugs (Draft for Comments) were revised and formed. Now the website of the center is publicized to listen to the opinions and suggestions of all walks of life.
We sincerely welcome valuable comments and suggestions from all walks of life on the draft for comments, and please give us feedback in a timely manner for subsequent improvement. Thank you for your participation and great support!
Publicity period: one month from the date of publicity
Feedback email: yshchzhn@cde.org.cn
Center for Drug Evaluation of the National Medical Products Administration
September 25, 2023