XHR.BIO 信合瑞创新药用辅料2025年08月21日 14:14天津

Feature
Calcium hydrogen phosphate dihydrate is soluble in dilute hydrochloric acid, dilute nitric acid, and acetic acid, slightly soluble in water, and insoluble in ethanol. It remains stable in air but loses its water of crystallization upon heating to 75°C, converting into the anhydrous form. Due to its excellent compressibility and flowability, it is widely used as a filler in pharmaceutical tablets.
Incompatibility of drugs in combination
Calcium hydrogen phosphate dihydrate should not be combined with tetracycline antibiotics. There have been reports of incompatibility with indomethacin, aspirin, aspartame, cefalexin, ampicillin, and erythromycin. Its surface is alkaline, making it unsuitable for use with alkali-sensitive drugs.
product application
Calcium hydrogen phosphate dihydrate is widely used in tablet formulations, serving both as an excipient and a calcium supplement. It is one of the most extensively utilized raw materials in nutraceutical and health products. Due to the excellent compressibility and flowability of its coarse particles, it is also commonly used in pharmaceutical preparations.
The primary deformation mechanism of coarse-grained calcium hydrogen phosphate dihydrate is brittle fracture, which reduces its strain sensitivity and facilitates the transition from laboratory-scale development to industrial production.
However, due to its high abrasiveness, lubricants such as 1% (w/w) magnesium stearate or 1% (w/w) sodium stearyl fumarate must be used during tableting. In the pharmaceutical industry, calcium hydrogen phosphate dihydrate is available in two particle size grades: milled products are mainly used in wet or dry granulation processes, while unmilled or coarse-grained products are primarily used in direct compression.
Performance indicators
01Electron micrograph
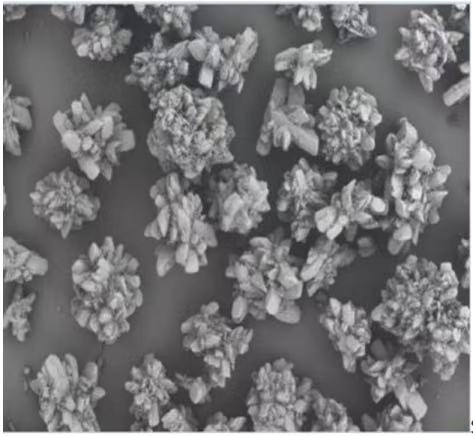
02Other parameters
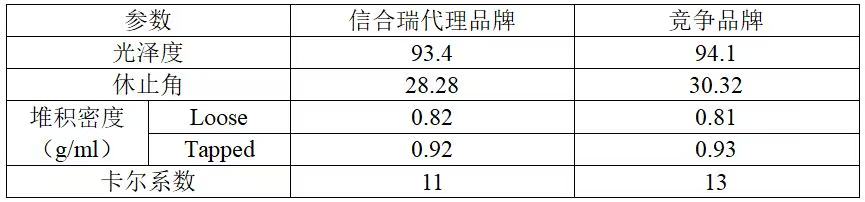
03Advantage
1.Free of metallic elements such as mercury, copper, nickel, and vanadium;
2.Cadmium, lead, and arsenic content < 1.0 ppm;
3.Excellent flowability and low friability, suitable for direct compression without the need for binders;
4.Registered with CDE under status “A“.
Application of dosage forms
Sample Request
Tianjin XHR Biotechnology Co.,LTD is a premium supplier for multiple renowned pharmaceutical enterprises, universities, and R&D institutions across China. We specialize in providing high-quality imported pharmaceutical excipients to the Chinese pharmaceutical industry. Our product portfolio includes nearly a hundred varieties of premium-grade pharmaceutical excipients, including critical injection-grade excipients and high-quality oral formulation excipients, widely used in tablets, capsules, suspensions, injections, and other dosage forms.

