Introduction
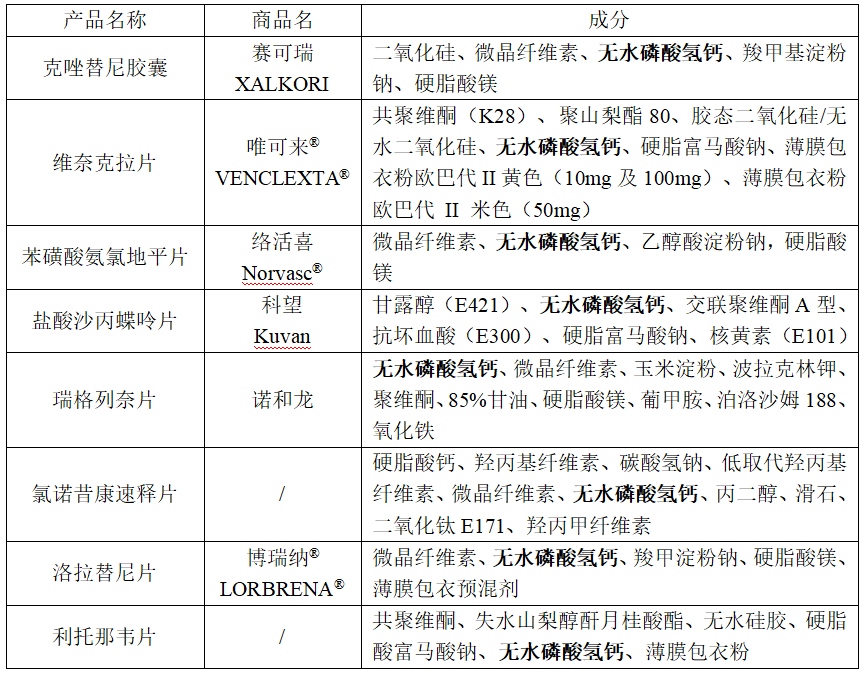
Calcium hydrogen phosphate is an excellent inorganic filler with outstanding compressibility. Its unique particle size, morphology, and density contribute to exceptional flowability even on high-speed tablet presses.
Calcium Hydrogen Phosphate: Also known as dicalcium phosphate, it is a white or off-white crystalline powder. Its chemical formula is CaHPO₄, and it exists in two forms: anhydrous calcium hydrogen phosphate (CaHPO₄) and dihydrate calcium hydrogen phosphate (CaHPO₄·2H₂O), with molecular weights of 136.06 and 172.1, respectively. It is highly stable and offers excellent biological availability of calcium and phosphorus, making it a high-quality calcium and phosphorus supplement. The dihydrate form contains no less than 18% phosphorus and 21% calcium. It crystallizes in the monoclinic system and loses its water of crystallization when heated to 80°C, converting into the anhydrous form, which has a triclinic crystal structure. It is odorless, slightly soluble in water, soluble in dilute hydrochloric and nitric acids, and sparingly soluble in dilute acetic acid.
Functions
👉Anhydrous calcium hydrogen phosphate can be used both as an excipient and as a calcium supplement. Due to the excellent compressibility and flowability of
👉Its coarse particles, it is also suitable for use in pharmaceutical preparations.
👉It facilitates easy scale-up from laboratory to industrial production. It serves as an excellent inorganic diluent, absorbent, and filler in solid dosage forms.
👉The primary deformation mechanism of coarse-grained anhydrous calcium hydrogen phosphate is brittle fracture, which reduces its strain sensitivity and further supports seamless transition from lab-scale to industrial applications.
👉When partially neutralized stearic acid is combined with 5–15 times its weight of an aqueous liquid, it forms a cream base.
👉With its superior adsorption capacity, it can powderize oily substances and extracts. It acts as both a filler and a binder in traditional Chinese medicine tablets, effectively adsorbing volatile oils and extracts.
Caution with contraindications
👈Calcium phosphates are incompatible with tetracycline. The surface of milled anhydrous calcium hydrogen phosphate is alkaline; therefore, it should not be used in combination with drugs that are sensitive to alkaline pH.
👈Studies have shown that milled and unmilled anhydrous calcium hydrogen phosphate differ in surface acidity and alkalinity: the surface of unmilled particles is acidic. This property is highly significant for improving drug stability, particularly when using a heavy compaction process, such as transitioning from roller compaction granulation to direct powder compression, where adjusting its particle size is advisable.
Advantages
1 Manufactured in a strict GMP-compliant environment, ensuring the production process is free from contamination by microorganisms, organic impurities, inorganic impurities, and residual pollutants.
2 High purity level of 98.0%–105%, with every batch undergoing microbial testing and additional specific analyses.
3 Exhibits excellent compressibility and low friability, making it suitable for direct compression applications, including with moisture-sensitive active ingredients.
4 Available in multiple grades to meet various API application requirements.
5 Registered with CDE under status “A“.
6 Shelf life of up to 5 years.
Formulation product application