In the field of pharmaceutical excipients, a disintegrant is an ingredient added to a formulation to prompt the rapid disintegration of the preparation into small units and accelerate the dissolution of the drug.When a disintegrant comes into contact with water, gastric juice or intestinal fluid, it absorbs the liquid to swell, dissolve or form a gel, which causes the structural disruption and disintegration of the preparation and promotes drug dissolution. Disintegration is the first step for drug dissolution and subsequent therapeutic effect exertion. After a drug is formulated into a solid preparation, its dissolution in water requires a certain period of time, which thus limits the drug absorption rate.To ensure the optimal therapeutic effect of solid preparations, disintegrants are generally incorporated, except for tablets designed for sustained drug release such as buccal tablets, sublingual tablets, implants and prolonged-release tablets. An ideal disintegrant should enable a tablet to disintegrate into granules and further disperse into the fine powder state prior to granulation.
After oral solid preparations enter the human gastrointestinal tract, they need to disintegrate rapidly and release active pharmaceutical ingredients to ensure absorption efficiency, and the achievement of this process relies on high-efficiency disintegrants. As a widely used disintegrant, croscarmellose sodium plays a pivotal role in tablets and capsules.
Croscarmellose sodium is a type of cross-linked cellulose ether and is included in the Chinese Pharmacopoeia (2025 Edition).It is a cross-linked polymer obtained by the cross-linking of sodium carboxymethyl cellulose.When this substance comes into contact with water, its volume swells rapidly to 4-8 times its original volume, and it exhibits capillary action and excellent water absorption and swelling properties.As a disintegrant, croscarmellose sodium is characterized by good compressibility and strong disintegrating power.When used as a disintegrant for tablets, it is suitable for both the wet granulation tableting process and the dry direct compression tableting process, and it is one of the commonly used super disintegrants at present.
Croscarmellose sodium is generally considered non-toxic and non-irritating.
It is listed in the Inactive Ingredient Guide of the FDA (for oral capsules and tablets).
Oral administration of large doses may cause a laxative effect, but the dosage used in the formulation of solid preparations rarely leads to this issue.
It has also been included in the USP/NF, BP, JP and PhEur.
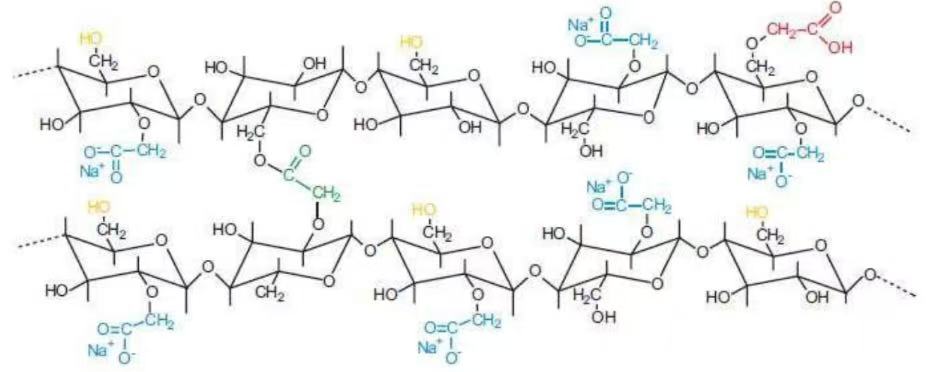

Sodium carboxymethyl cellulose (CCS) is used as a disintegrant in oral dosage forms such as capsules, tablets, and granules. In tablet formulations, CCS is suitable for both direct compression and wet granulation compression processes. During wet granulation, CCS can be added either at the wetting stage or the drying stage (intragranular and extragranular addition) to optimally utilize the capillary and swelling effects of the disintegrant. When used as a disintegrant, the concentration of CCS can be up to 5% (w/w). However, the typical usage level is 2% (w/w) for direct compression and 3% (w/w) for wet granulation compression.
Incompatibilities:
In both wet granulation and direct compression processes, the presence of hygroscopic excipients (such as sorbitol) can slightly reduce the efficiency of disintegrants like CCS.
CCS is incompatible with strong acids, iron, or soluble salts of other metals (e.g., aluminum, mercury, zinc).
Experimental Case Study
Materials
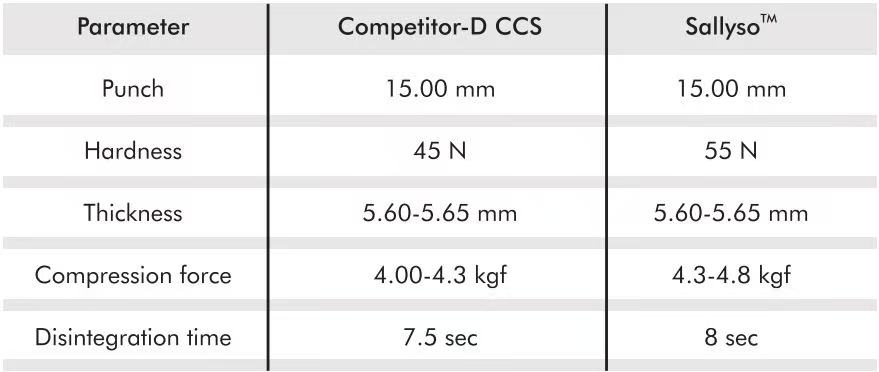
- Sallyso™ is a CCS product from Gangwal, and one commercially available CCS product (Competitor-D CCS) was also selected for comparison. The usage level was 2.1%.
- Silicified microcrystalline cellulose was used as the filler at 26.6%.
- Sodium stearyl fumarate was used as the lubricant at 0.5%.
- Performance comparison was conducted based on CCS from different manufacturers.
Method
- Metformin hydrochloride tablets were prepared by direct compression, 850 mg, φ15 mm.
- Tablet thickness and hardness were evaluated using a hardness tester.
- Disintegration time was evaluated using a disintegration tester.
- Accelerated conditions: Storage for 6 months at 40°C/75% RH in open containers.
Results and Conclusion
The disintegration performance of Sallyso™ CCS was comparable to that of Competitor-D CCS.
Product Advantage
- Fast disintegration
- Superior dissolution performance
- Excellent bioavailability of active ingredients
- No adverse effect on tablet compression performance and material flowability
- Cost-effective (usage level only 2.0–6.0% w/w)
- Application scenarios: Wet granulation, dry granulation, direct compression
- CDE Registration Number: F20230000371

